Charles law gives the relationship between volume of gas and temperature.
It states that for a fixed amount of gas at constant pressure, volume of gas is directly proportional to the temperature.
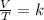
where V - volume and T - temperature and k - constant
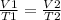
where parameters for the first instance are on the left side and parameters for the second instance are on the right side of the equation
T1 - temperature in Kelvin , -206.6 °C + 273.15 = 66.55 K
T2 - 175.9 °C + 273.15 = 449.05 K
substituting the values in the equation
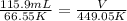
V = 782 mL
the new volume is 782 mL