Answer : The root-mean-square speed of methane gas is, 739.7 m/s
Solution :
Formula used :
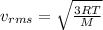
where,
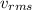 = root mean square speed
= root mean square speed
R = gas constant =
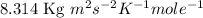
T = temperature of gas =
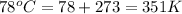
M = molecular weight of methane = 16 g/mole = 0.016 Kg/mole (1 Kg = 1000 g)
Now put all the given values in the above formula, we get the rms speed.
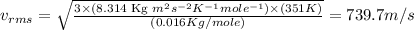
Therefore, the root-mean-square speed of methane gas is, 739.7 m/s