Answer:
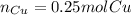
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:

In such a way, since it is already balanced, we develop the following stoichiometric dimensional analysis to find the moles of copper needed to react with sulfur to produce 0.24 moles of copper sulfide taking into account they have a 1 to 1 relationship, as shown below:
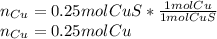
Best regards.