The half-life is the time the sample takes to reduce to half of its original value. If we call
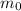
the initial mass of the sample, this means that after 1 half-life the mass will be
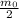
, after 2 half-lives the mass will be
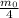
, and so on..
Therefore, after x half-lives the mass of the sample will be
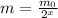
(1)
In our problem, the initial mass is
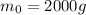
while the mass after x half-lives is
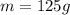
, so by using equation (1) we can find the value of x:
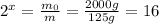
From which
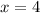
And the correct answer is C).