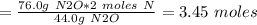Answer:
Moles of N = 3.45
Step-by-step explanation:
Given:
Mass of N2O = 76.0 g
To determine:
Moles of N present in the given mass of N2O
Step-by-step explanation:
Based on the formula stoichiometry:
1 mole of N2O contains 2 moles of N
Now, 1 mole of N2O = 44.0 g (i.e. the molar mass of N2O)
44.0 g of N2O contains 2 moles of N
Therefore, moles of N in 76.0 g of N2O would be:
=