Since the cation (A) has a charge twice in magnitude than that of the anion (X), you can say the magnitude of the charge of the cation is 2c, while the magnitude of the charge of the anion is c.
Also remember that cations are positive ions, so the charge would be +2c, and that anions are negative ions, so the charge would be -c.
A binary ionic compound of A and X would have to have a net charge of zero. If A has a +2c charge, you would need two X anions to balance out every A cation, so the formula would be:
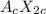
The simplest formula would be
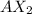
.