Answer:
The hydroxide concentration in the solution is 0.0063 M.
Step-by-step explanation:
The pH of the solution is defined as negative logarithm of
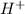 concentration.
concentration.
![pH=-\log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/vwilut25e4cux34589pwoorivy6w6y51xe.png)
The pH of the ammonia solution is 11.8
pH + pOH = 14
pOH = 14 - 11.8 = 2.2
The pOH of the solution is defined as negative logarithm of hydroxide ion concentration.
![pOH=-\log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/high-school/ur2f3m6zoirj5p05ac4nknmpiip97f0mi9.png)
![2.2=-\log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/high-school/clfvfsips3jyuvwos0zdbs1rkhjuu78ya4.png)
![[OH^-]=0.0063 M](https://img.qammunity.org/2019/formulas/chemistry/high-school/2mkxf4b3nbl9kz4lwxevzup86av347qvtp.png)
The hydroxide concentration in the solution is 0.0063 M.