Answer:
mass of water is 225g
Step-by-step explanation:
What is the mass of water required to prepare 250.0 g of 10.0% copper(ii) sulfate solution?
Note:mass is the quantity of water in a body.
The question is asking us to look for the amount of water in the solution that contains 10% of copper (ii) sulphate vi by mass
percentage concentration by mass=mass of solute/mass of solution*100%
c%=
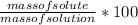
aslo
mass of solution=mass of solute+mass of solvent
the solvent in this case is water
the solute is copper (ii) tetraoxosulphate (vi)
10%=mass of solute/250*100%
mass of solute=25g
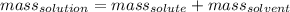
250g=25g+mass of solvent
mass of solvent=250-25
mass of solvent=225g
mass of water is 225g