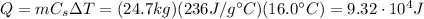The amount of heat needed to increase the temperature of a substance by

is given by
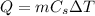
where
m is the mass of the substance
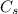
is its specific heat capacity

is the increase of temperature
The sample of silver of our problem has a mass of
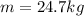
. Its specific heat capacity is
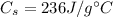
and the increase in temperature is
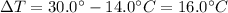
Therefore, the amount of heat needed is