The energy required to melt one gram of ice at 0 degrees is given by:
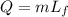
where m is the mass of the ice and
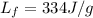
is the latent heat of fusion of ice. Substituting, we find
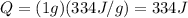
The energy required to boil one gram of water at 100 degrees is given by:
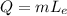
where m is the mass of the water and
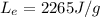
is the latent heat of evaporation of the water. Substituting, we find
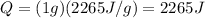
Therefore, it is required more energy to boil one gram of water at 100 degrees than to melt one gram of ice at 0 degrees.