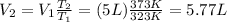To solve the problem, we can use Charle's law, which states that for an ideal gas at constant pressure the ratio between absolute temperature T and volume V remains constant:
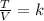
For a gas transformation, this law can be rewritten as
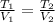
(1)
where 1 and 2 label the initial and final conditions of the gas.
Before applying the law, we must convert the temperatures in Kelvin:
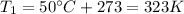
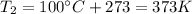
The initial volume of the gas is
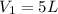
, so if we re-arrange (1) we find the new volume of the gas: