Answer:
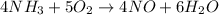
Explanation: The given equation is:
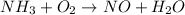
Let's balance all the atoms one by one:
Both sides have equal number of N atoms so let's move to the next atom that is H.
There are three H on left side and two H atoms on right side. To balance H, we need to multiply left side by 2 and right side by 3. On doing this, the equation would look as:
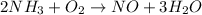
Now, left side has two N where as right side has only one N. So, to balance this for N, we need to multiply right side(NO) by 2.
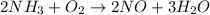
Above equation has two oxygen on left side and five oxygen on right side. To make this balance we need to multiply left side oxygen by 2.5.
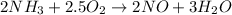
Since whole numbers are used as coefficients for balancing equations, let's multiply the whole equation by 2.
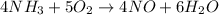
So, the finally balanced equation is
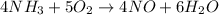 .
.