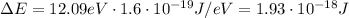The energy levels of the hydrogen atom are given by
![E=-13.6 (1)/(n^2) [eV]](https://img.qammunity.org/2019/formulas/physics/college/bli5tkhd72qktnn3b5rt9jsii67rmn3d6b.png)
where n is the level number.
For n=3 we have
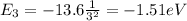
while for n=1 (ground state) we have
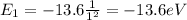
The energy of the emitted photon is equal to the energy difference between the two levels in the transition from n=3 to n=1:
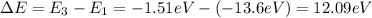
In joule, this corresponds to