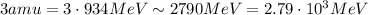The correct answer is:
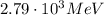
Let's see why.
1 amu corresponds to the mass of the proton, which is:
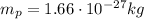
if we convert this into energy, using Einstein equivalence between mass and energy, we find:
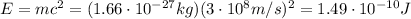
Now we can convert it into electronvolts:
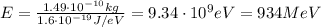
So, 1 amu = 934 MeV. Therefore, 3 amu corresponds to 3 times this value: