Hello there!
The chemical equation for the
decomposition reaction of KClO₃ is the following one:
2KClO₃ → 2KCl + 3O₂For determining the moles of KCl produced from 89,0 g of KClO₃ we are going to use the following conversion factor to go from grams of KClO₃ to moles of KCl:
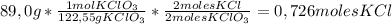
So,
0,726 moles of KCl are produced.
Have a nice day!