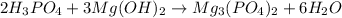Answer: X =
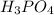 ; Y =
; Y =
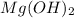
Explanation: When an acid reacts with the base then it undergoes neutralization to form a salt and water.
An acid is a substance that ionizes in the water to give hydrogen ion and have pH less than 7.
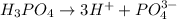
A base is a substance that ionizes in the water to give hydroxide ion and has pH more than 7.
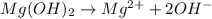
Salts are neutral compounds with pH equal to 7.