Hello there!
If we assume that the quartz rock is pure silicon dioxide, we only need to use a conversion factor to go from grams of quartz rock (SiO₂) to grams of Silicon. The conversion factor uses the molar mass of SiO₂ (
60,08 g/mol) and the atomic mass of Silicon (
28,0855 g/mol):
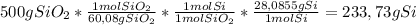
So,
233,73 grams of Silicon can be obtained.
Have a nice day!