Answer: The volume of water added must be, 1.42 L
Explanation : Given,
Mass of sodium nitrate = 5.40 g
Concentration of solution = 3.81 g/L
3.81 g/L concentration means that, 3.81 grams of sodium nitrate present in 1 liter of water.
As, 3.81 grams of sodium nitrate present in 1 liter of water
So, 5.40 grams of sodium nitrate present in
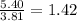 liter of water
liter of water
Therefore, the volume of water added must be, 1.42 L