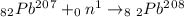Answer:- The answer is choice C. Lead-208.
Explanations:- In nuclear reaction a neutron is symbolized as
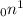 . From it's symbol it is clear that atomic number is 0 and mass number is 1. So, when a neutron is consumed by a radioactive element then it's atomic number remains same but the mass number increases by one unit.
. From it's symbol it is clear that atomic number is 0 and mass number is 1. So, when a neutron is consumed by a radioactive element then it's atomic number remains same but the mass number increases by one unit.
Atomic number remains unchanged means the product atom is still lead but the new mass number of the lead isotope is = 207 + 1 = 208
The equation could be written as: