Answer:- 3.83 g of hydrogen gas are formed.
Solution:- It is a stoichiometry problem and could easily be solved using dimensional analysis. The balanced equation for the decomposition of ammonia gas to give nitrogen and hydrogen gases is:
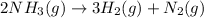
From balanced equation, there is 2:3 mol ratio between ammonia and hydrogen. We start with given grams of ammonia and convert them to moles on dividing the grams by molar mass.
In next step the moles of ammonia are multiplied by mol ratio to get the moles of hydrogen which are finally multiplied by it's molar mass to get the grams of hydrogen gas formed.
The set is shown below:
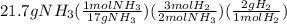
=
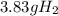
So, from the calculations 3.83 g of hydrogen gas are formed by the decomposition of 21.7 g of ammonia gas.