Step-by-step explanation:
Raoult's law for volatile solutes states that at a given temperature vapor pressure of a component is equal to the mole fraction of that component component in the solution multiplied to the vapor pressure of the component in the pure state.
Mathematically,
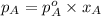
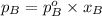
P =
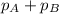
=
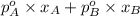
Since,
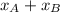 = 1
= 1
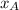 = 1 -
= 1 -
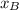
hence, P =
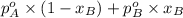
P =
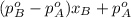
On the other hand, Raoult's law for non-volatile solutes states that relative lowering of vapor pressure of a solution that has non-volatile solute is equal to the mole fraction of the solute in the solution.
Mathematically,
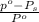 =
=
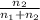 =
=
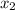
where,
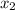 = mole fraction of solute
= mole fraction of solute
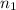 = moles of solvent
= moles of solvent
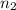 = moles of solute
= moles of solute
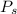 = vapor pressure of the solution
= vapor pressure of the solution
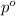 = vapor pressure of pure solvent
= vapor pressure of pure solvent