Hello!
To solve this question we are going to use the
Avogadro's Number, which tells us how many molecules of a given substance are in a mole. The Avogadro's number is
6,02*10²³ molecules/mol, and we are going to use this value in the following conversion factor:
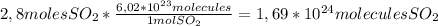
So, there are 1
,69*10²⁴ molecules of SO₂ in 2,8 moles.
Have a nice day!