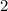Answer:
Oxygen's valence is 2
Step-by-step explanation:
Given -
Atomic number of oxygen is 8
The electrons at second energy level must be 8 (8 electron signifies a complete octate/stable state)
It is given that there are 6 electron at second energy level, thus number of electrons required to reach a stable state is equal to
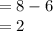
Since, oxygen needs two electron thus it will act as receiver and hence, it valence will be equal to