Hello!
The balanced chemical equation for the reaction between sodium and chlorine is the following:
2Na + Cl₂ → 2NaClTo know the mass of Sodium Chloride produced, we are going to use the following conversion factor to go from grams of Chlorine gas to grams of Sodium Chloride:
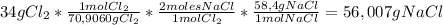
So,
56,007 g of NaCl are produced
Have a nice day!