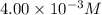Answer : The correct option is, (2)
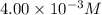
Step-by-step explanation:
pH : It is defined as the negative logarithm of hydrogen ion or hydronium ion concentration.
The mathematical expression is :
![pH=-\log [H^+]](https://img.qammunity.org/2019/formulas/physics/high-school/iidawris7irvi0bu33z0a9xjzagtaknk6o.png)
or,
![pH=-\log [H_3O^+]](https://img.qammunity.org/2019/formulas/chemistry/college/kv1d6x7k5u8rrmnok6mdbtm4oardzajzce.png)
Now we have to calculate the
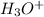 concentration.
concentration.
![pH=-\log [H_3O^+]](https://img.qammunity.org/2019/formulas/chemistry/college/kv1d6x7k5u8rrmnok6mdbtm4oardzajzce.png)
![2.4=-\log [H_3O^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/k82rb3j8wglq7lufeaak9rpnnohvg3ivrq.png)
![[H_3O^+]=3.98* 10^(-3)M\approx 4.00* 10^(-3)M](https://img.qammunity.org/2019/formulas/chemistry/high-school/kb5dj72fcwiwb2lhprvj9ftn25bo7ta05r.png)
Therefore, the
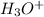 concentration is,
concentration is,