Answer:
Paramagnetic
Step-by-step explanation:
Paramagnetic are those which has unpaired electrons and diamagnetic are those in which all electrons are paired.
Given the atomic number - 71
The electronic configuration is -
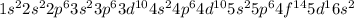
The electron in 5d orbital = 1
This 1 electron will be unpaired as electron is first singly filled.
Thus, the element is paramagnetic as the electron is unpaired.