Answer:
The mass of carbon dioxide that is added to the atmosphere is 1.50526 kg.
Step-by-step explanation:
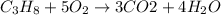
Mass of octane = 1.3 kg = 1300 g
Moles of octane =
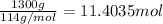
According to reaction 1 mole of octane gives 3 moles of carbon dioxide.
Then 11.4035 moles of octane will producer:
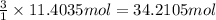 of carbon dioxide
of carbon dioxide
Mass of 34.2105 moles of carbon dioxide:
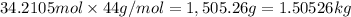
The mass of carbon dioxide that is added to the atmosphere is 1.50526 kg.