Answer:
![[Zn(H_2O)_2(CO)_2][PdBr_4]](https://img.qammunity.org/2019/formulas/chemistry/college/gi4b0qv652ey5t7nrj0fje1ng3w5qmt5kr.png)
Step-by-step explanation:
Hello,
In this case, one could build-up the formula, via analyzing the name as follows:
- It says tetrabromopalladate (IV), it means that it is a mixed anion, composed by four bromine atoms (tetrabromo) and one palladium atom due suffix ate), therefore the anion is
![[PdBr_4]](https://img.qammunity.org/2019/formulas/chemistry/college/rwqsg4qzgrb346nhwntd6635ru6kbjmmnh.png) .
.
- By means of the cation, it starts by diaqua, so it has two
 as an aquocation. Next, is says dicarbonyl, so two carbonyl groups
as an aquocation. Next, is says dicarbonyl, so two carbonyl groups
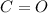 are bonded as well. Finally, the metallic part is given by the presence of zinc, so the cation turns out as
are bonded as well. Finally, the metallic part is given by the presence of zinc, so the cation turns out as
![[Zn(H_2O)_2(CO)_2]](https://img.qammunity.org/2019/formulas/chemistry/college/jgya0t3syof4gvlj5uil2870t27lvlefco.png) by complexity.
by complexity.
Therefore, the resulting formula is:
![[Zn(H_2O)_2(CO)_2][PdBr_4]](https://img.qammunity.org/2019/formulas/chemistry/college/gi4b0qv652ey5t7nrj0fje1ng3w5qmt5kr.png)
Best regards.