Hello!
There is a useful relationship that we can use to determine the number of moles of an ideal gas at
Standard Temperature and Pressure (0 °C; 1 bar).
It says that
1 mole of an ideal gas occupies 22,4 L. Knowing that we can apply the following conversion factor to go from mL of CO₂ to moles of CO₂
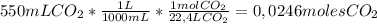
So, the number of moles contained in 550 mL of Carbon Dioxide at STP is
0,0246 molesHave a nice day!