Step-by-step explanation:
When an acid reacts with a base then it results into the formation of a salt and water. This type of chemical reaction is known as neutralization reaction.
For example,

Here, HCl is the acid and KOH is the base.
So, hydrogen ion from HCl will combine with hydroxide ion of KOH to result in the formation of water (
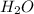 ). Whereas chlorine ion from HCl will combine with potassium ion of KOH to result in the formation of KCl.
). Whereas chlorine ion from HCl will combine with potassium ion of KOH to result in the formation of KCl.