Answer: B
Step-by-step explanation:
Isotopes are elements which have same atomic number but different mass number.
General representation of an element is given as:
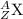
where,
Z represents Atomic number
A represents Mass number
X represents the symbol of an element
Thus for helium , which is shown to be
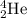
Mass number is defined as the sum of number of protons and neutrons that are present in an atom.
Mass number = Number of protons + Number of neutrons = 4
Number of neutrons = 4-2 =2
Atomic number is defined as the number of protons or number of electrons that are present in an atom.
Atomic number = Number of electrons = Number of protons = 2
Thus the representation with 2 protons and 2 neutrons will be the most common isotope of helium.