Hello!
The chemical
neutralization reaction between NaOH and H₂SO₄ is the following:
2NaOH(aq) + H₂SO₄(aq) → Na₂SO₄(aq) + 2H₂O(l)So, we can apply the molar equivalence at the endpoint in the following way:
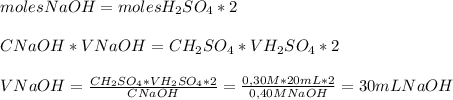
So,
30 mL of NaOH are required to reach an endpoint
Have a nice day!