Answer:
Argon, neon and xenon are examples of noble gases.
C is correct option.
Step-by-step explanation:
According to electronic configuration
Argon:
The atomic number of argon is 18.
The electronic configuration of argon
Ar= 2,8,8
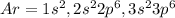
Neon:
The atomic number of argon is 10.
The electronic configuration of argon
Ar= 2,8
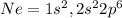
Xenon:
The atomic number of argon is 54.
The electronic configuration of argon
Ar= 2,8,18,18,8
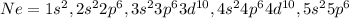
Argon, neon and xenon are examples of noble gases because the last shell is completely filled and they does not participate in any reaction so, these atoms are called noble gasses.
Hence, Argon, neon and xenon are examples of noble gases.