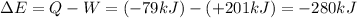The variation of internal energy of a system is given by
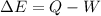
where
Q is the heat absorbed by the system
W is the work done by the system
It's important to keep in mind the correct sign convention when using the previous equation:
Q is positive if absorbed by the system, negative if released by the system
W is positive if done by the system on the surrounding, negative if done by the surrounding on the system
In this problem, the work is done by the system, so W=+201 kJ, and the heat is lost by the system, so Q=-79 kJ, therefore the variation of internal energy is