Answer:
a.
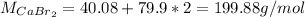
b.
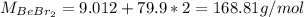
c.
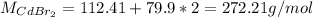
d.
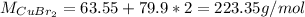
Step-by-step explanation:
Hello!
In this case, since the molar mass of a compound is computed by adding the molar mass of each element composing it and multiplying it by the number of atoms in the molecule, we have:
a.
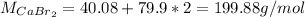
b.
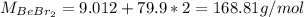
c.
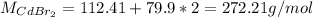
d.
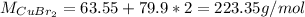
Best regards!