Answer:
34.02 g.
Step-by-step explanation:
Hello!
In this case, since the gas behaves ideally, we can use the following equation to compute the moles at the specified conditions:
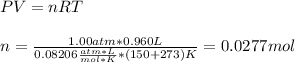
Now, since the molar mass of a compound is computed by dividing the mass over mass, we obtain the following molar mass:
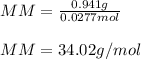
So probably, the gas may be H₂S.
Best regards!