Pressure and Volume are related by Boyle's Law as:
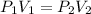
P1 = Initial pressure = 1.25 atm
V1 = Initial volume = 2.50 L
P2 = Pressure at ocean bottom = 95 atm
V2 = Volume at ocean bottom = x = ?
Using the values in the equation, we get
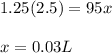 This means, the volume at the bottom of the ocean will be 0.03 L. The volume is decreased due to increased pressure.
This means, the volume at the bottom of the ocean will be 0.03 L. The volume is decreased due to increased pressure.