Step-by-step explanation:
Heat is equal to heat capacity multiplied by change in temperature.
Mathematically,
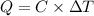
where C = heat capacity
 = change in temperature
= change in temperature
Therefore, calculate heat as follows.
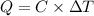
=
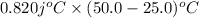
= 20.5 j
Thus, we can conclude that heat is required to raise the temperature of the cup is 20. 5 joules.