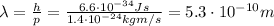(a) for a photon, the relationship between frequency f and energy E is
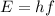
(1)
where h is the Planck cosntant.
First, we need to convert the energy from eV to Joule:
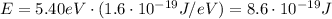
Then, using (1) we can find the frequency of the photon:
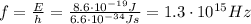
and finally we can find its wavelength:
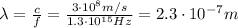
(b) now we have to find the De Broglie wavelength of an electron with same energy of the previous photon. Again, we must convert the energy in Joules first:
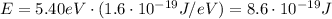
Then we can use the relationship between momentum p and energy E of a particle, to find p: (electron mass:
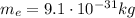
)
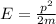
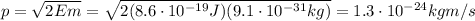
And finally we can use De Broglie relationship to find the wavelength of the electron: