Hello!
The pKa of an indicator tells us the pH where it changes color. The pKa of Methyl red is
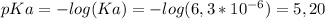
So, at a pH=7,8,
Methyl Red would be at a pH over its pKa, so it would be in its basic form. The Basic Form of Methyl Red is Yellow, so
the solution would be Yellow.Have a nice day!