Hello!
To solve this problem, we will use the
Boyle's Law, which describes how pressure changes when volume changes and vice-versa. The equation for this law is the following one, and we'll clear for V2:
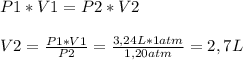
So, the final volume after increasing the pressure would be
2,7 L. That means that volume decreases when the pressure increases
Have a nice day!