Hello!
The dissociation reaction of HCl is the following:
HCl(aq) + H₂O(l) → H₃O⁺(aq) + Cl⁻(aq)The concentration of H₃O⁺ is the same concentration as HCl (0,0035 M). Now, we calculate the pH using the following equation:
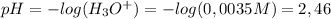
Now, we determine the
pOH using the following equation:
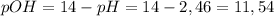
To finish, we apply the
definition of pOH and clear for [OH⁻]:
![[OH]= 10^(-pOH)= 10^(-11,54)=2,88*10^(-12) M](https://img.qammunity.org/2019/formulas/chemistry/middle-school/uz7ozcrnjy3xuk1e75xplj2h1p5hxmjgkm.png)
So,
[OH⁻]=2,88*10⁻¹²
Have a nice day!