Answer:
The correct option is a
Step-by-step explanation:
In the reaction, 2 moles of Aluminium (Al) reacted with 3 moles of Iron (II) oxide (FeO) to produce Iron (Fe) and Aluminium oxide (Al₂O₃) in the ratio 3:1 respectively. From the above, it can be deduced that when 3 moles of iron is produced, 1 mole of aluminium oxide is produced, hence when 0.6 mole of iron is produced, X mole of aluminium oxide is produced, where X is the unknown.
3 ⇒ 1
0.6 ⇒ X
X =
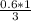
X = 0.2 mole of Al₂O₃ will be produced when 0.60 mole of Fe is produced in the reaction (in the question)