Step-by-step explanation:
It is known that an alpha particles is basically a helium nucleus and it contains 2 protons and 2 neutrons.
Symbol of an alpha particle is
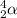 or
or
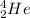 .
.
Therefore, when
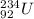 undergoes alpha decay then the reaction will be as follows.
undergoes alpha decay then the reaction will be as follows.
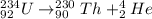
Hence, the resulting element formed is thorium.
Therefore, we can conclude that atomic number of the resulting element is 90.