Answer:- Molarity of the solution is 10 M.
Solution:- Molarity is a way to express the concentration of the solution and it is moles of solute per liter of the solution.
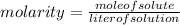
From given info, 0.5 moles of Lithium chloride are dissolved in 0.05 L of water.
So, molarity of the solution =
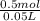 = 10M
= 10M
(where M stands for molarity).