Answer:
- Addition of NaH releases hydrogen gas.
- Addition of
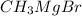 releases methane.
releases methane.
Step-by-step explanation:
Hello,
On the attached picture, you will find both reactions as shown below:
- Addition of NaH: in this case, due to the fact that the alkynes are slightly acid, they are able to donate one proton, thus, the hydrogen bonded with the triple-bonded carbon will be released as hydrogen gas.
- Addition of
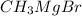 : in this case, a Grignard reactant, magnesium methyl bromide, is added so the methyl from such reactant will accept the hydrogen bonded with the triple-bonded carbon, forming a grignard intermediate and methane gas.
: in this case, a Grignard reactant, magnesium methyl bromide, is added so the methyl from such reactant will accept the hydrogen bonded with the triple-bonded carbon, forming a grignard intermediate and methane gas.
Best regards.