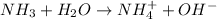Step-by-step explanation:
According to Bronsted-Lowry an acid is defined as the specie which is able to donate hydrogen ions when dissolved in water.
For example,
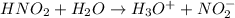
Chemical formula of nitrous acid is
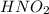 . Since, it donates a hydrogen ion in water hence, it acts as a Bronsted-Lowry acid.
. Since, it donates a hydrogen ion in water hence, it acts as a Bronsted-Lowry acid.
On the other hand, bases are the species which are able to accept hydrogen ions when dissolved in water.
For example,