Answer:
Step-by-step explanation:
18 gram of water contains 2 g of hydrogen
3.123 gram of water will contain 2 x 3.123 / 18 = .347 g of hydrogen .
44 gram of carbon dioxide contains 12 g of carbon
7.691 gram of carbon dioxide will contain 12 x 7.691 / 44 = 2.1 g of carbon .
So the sample will contain 2.912 - ( .347 + 2.1 ) g of oxygen .
= .465 g of oxygen .
moles of Carbon = 2.1 / 12 = .175
moles of hydrogen = .347 / 1 = .347
moles of oxygen = .465 / 16 = .029
Ratio of moles of carbon , oxygen and hydrogen ( C,O,H )
= 0.175 : 0.029 : 0.347
= .175/ .029 : 1 : .347 / .029
= 6 : 1 : 12
So empirical formula = C₆H₁₂O
Let the molecular formula be
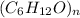
molecular weight = n ( 6 x 12 + 12x 1 + 16)
= 100 n
Given 100 n = 100.1
n = 1
Molecular formula = C₆H₁₂O.