Answer:
Molarity of aqueous solution = 0.14 M
Step-by-step explanation:
Molarity is defined as the number of moles of solute present in 1 L of the solution.
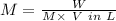
Where,
W = Mass of the solute
M = Molecular mass of the solute
V = Volume of the solution
Volume of the solution = 1.5 L
Mass of glucose = 40.0 g
Molecular mass of glucose = 180.16 g/mol
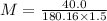
= 0.1480 M
= 0.14 M