Answer:
Step-by-step explanation:
Given that:
2H₂(g) + 2NO(g) → 2H₂O(g) + N₂(g)
Experiment [H₂] (M) [NO] (M) Initial Rate (M/s)
1 0.010 0.025 2.4 × 10⁻⁶
2 0.0050 0.025 1.2 × 10⁻⁶
3 0.010 0.0125 0.60 × 10⁻⁶
The general formula for rate law is:
![rate = k[H_2]_x [NO]_y](https://img.qammunity.org/2022/formulas/chemistry/college/xtg3mppfg6bsp9jj4dvxgljw1xezesluyv.png)
From (1) and (2), it is obvious that the concentration of NO looks constant unlike that of H₂ which has decreased by 1/2. Similarly, the initial rate also reduced by 1/2. Hence, the initial rate is proportional to the concentration of H₂. So, x = 1
Also;
From (1) and (3), it is obvious that the concentration of H₂ looks constant unlike that of NO which has decreased by 1/2. Similarly, the initial rate also reduced by 1/4. Hence, the initial rate is proportional to the concentration of NO. So, y = 2
∴
The overall rate law is:
![rate = k[H_2][NO]_2](https://img.qammunity.org/2022/formulas/chemistry/college/2b2hen2yeifh9wcp56065dgo6b5vefmq2h.png)
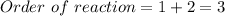
(b)
From (1)
The rate constant is:
![rate = k[H_2][NO]_2](https://img.qammunity.org/2022/formulas/chemistry/college/2b2hen2yeifh9wcp56065dgo6b5vefmq2h.png)
∴
![k = (rate)/( [H_2] [NO]^2)](https://img.qammunity.org/2022/formulas/chemistry/college/khwfg4sleet7mijvp74e3bubtxd0698i4h.png)
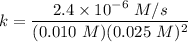
k = 0.38 / M².s
(c)
From the rate law, it is pertinent to understand that the slow step in the reaction includes one molecule of H₂ and two molecules of NO, where O atoms serve as an intermediary.
SO;
H₂ + 2NO → N₂ + H₂O + O slow step
O + H₂ → H₂O fast step
2H₂ + 2NO → 2H₂O + N₂